What are Valence electrons? How do they relate to the periodic table?
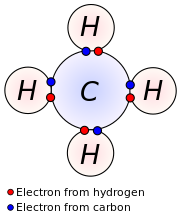
Iv covalent bonds. Carbon has four valence electrons and hither a valence of 4. Each hydrogen atom has one valence electron and is univalent.
In chemical science and physics, a valence electron is an electron in the outer shell associated with an atom, and that can participate in the formation of a chemical bond if the outer shell is not airtight; in a unmarried covalent bond, both atoms in the bond contribute one valence electron in order to form a shared pair.
The presence of valence electrons can decide the chemical element's chemical properties, such equally its valence—whether it may bond with other elements and, if and then, how readily and with how many. In this way, a given element'southward reactivity is highly dependent upon its electronic configuration. For a principal-group element, a valence electron can be only in the outermost electron shell; for a transition metal, a valence electron can also exist in an inner shell.
An atom with a closed beat of valence electrons (corresponding to a element of group 0 configuration) tends to be chemically inert. Atoms with ane or two valence electrons more than a airtight shell are highly reactive due to the relatively low energy to remove the extra valence electrons to form a positive ion. An atom with one or two electrons less than a closed vanquish is reactive due to its trend either to proceeds the missing valence electrons and form a negative ion, or else to share valence electrons and form a covalent bond.
Similar to a core electron, a valence electron has the power to absorb or release energy in the form of a photon. An energy gain can trigger the electron to move (bound) to an outer beat; this is known as atomic excitation. Or the electron can fifty-fifty pause costless from its associated cantlet'south beat out; this is ionization to course a positive ion. When an electron loses energy (thereby causing a photon to exist emitted), then it tin can motility to an inner beat which is not fully occupied.
Overview [edit]
Electron configuration [edit]
The electrons that determine valence – how an atom reacts chemically – are those with the highest energy.
For a main-group element, the valence electrons are defined equally those electrons residing in the electronic beat of highest principal breakthrough number n.[one] Thus, the number of valence electrons that it may have depends on the electron configuration in a simple way. For example, the electronic configuration of phosphorus (P) is 1s2 2s2 2phalf dozen 3s2 3p3 so that there are 5 valence electrons (3sii 3p3), corresponding to a maximum valence for P of 5 as in the molecule PF5; this configuration is unremarkably abbreviated to [Ne] 3s2 3piii, where [Ne] signifies the cadre electrons whose configuration is identical to that of the noble gas neon.
However, transition elements take partially filled (n−1)d free energy levels, that are very close in energy to the n south level.[2] So every bit opposed to chief-group elements, a valence electron for a transition metal is defined as an electron that resides outside a noble-gas core.[3] Thus, generally, the d electrons in transition metals behave as valence electrons although they are not in the outermost shell. For example, manganese (Mn) has configuration 1s2 2s2 2p6 3s2 3p6 4s2 3dfive; this is abbreviated to [Ar] 4s2 3dv, where [Ar] denotes a cadre configuration identical to that of the noble gas argon. In this cantlet, a 3d electron has energy similar to that of a 4s electron, and much higher than that of a 3s or 3p electron. In issue, there are possibly seven valence electrons (4stwo 3d5) outside the argon-like core; this is consequent with the chemical fact that manganese tin can have an oxidation state every bit loftier as +7 (in the permanganate ion: MnO −
4 ).
The further right in each transition metallic serial, the lower the free energy of an electron in a d subshell and the less such an electron has valence properties. Thus, although a nickel cantlet has, in principle, ten valence electrons (4s2 3dviii), its oxidation country never exceeds iv. For zinc, the 3d subshell is complete in all known compounds, although information technology does contribute to the valence band in some compounds.[iv]
The d electron count is an alternative tool for understanding the chemistry of a transition metal.
The number of valence electrons [edit]
The number of valence electrons of an element can be determined by the periodic tabular array group (vertical column) in which the element is categorized. With the exception of groups three–12 (the transition metals), the units digit of the group number identifies how many valence electrons are associated with a neutral atom of an element listed under that particular cavalcade.
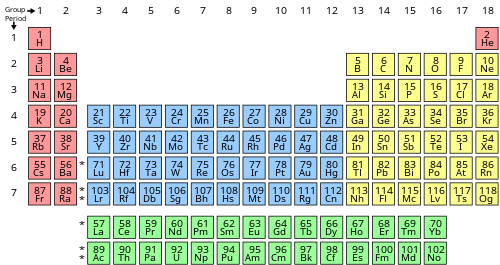
| Periodic tabular array block | Periodic table group | Valence electrons |
|---|---|---|
| due south | Group 1 (I) (brine metals) | i |
| Group 2 (II) (element of group i world metals) and helium | two | |
| f | Lanthanides and actinides | 3–sixteen[a] |
| d | Groups 3-12 (transition metals) | 3–12[b] |
| p | Grouping 13 (3) (boron group) | 3 |
| Grouping fourteen (IV) (carbon grouping) | 4 | |
| Group 15 (V) (pnictogens or nitrogen grouping) | 5 | |
| Group xvi (VI) (chalcogens or oxygen grouping) | half dozen | |
| Group 17 (Vii) (halogens) | 7 | |
| Group 18 (8 or 0) (noble gases) except helium | viii |
- ^ Consists of ndue south, (due north−2)f, and (n−1)d electrons.
- ^ Consists of due norths, and (n−1)d electrons.
Helium is an exception: despite having a 1s2 configuration with two valence electrons, and thus having some similarities with the alkaline metal world metals with their ns2 valence configurations, its shell is completely full and hence it is chemically very inert and is usually placed in group 18 with the other noble gases.
Valence shell [edit]
The valence vanquish is the prepare of orbitals which are energetically attainable for accepting electrons to form chemical bonds.
For chief-grouping elements, the valence shell consists of the due norths and np orbitals in the outermost electron shell. For transition metals the orbitals of the incomplete (n−1)d subshell are included, and for lanthanides and actinides incomplete (north−2)f and (n−one)d subshells. The orbitals involved can be in an inner electron beat out and do not all correspond to the same electron shell or principal quantum number due north in a given element, but they are all at like distances from the nucleus.
| Element type | Hydrogen and helium | p-block (main-grouping elements) | d-block (Transition metals) | f-block (Lanthanides and actinides) |
|---|---|---|---|---|
| Valence orbitals[5] |
|
|
|
|
| Electron counting rules | Duet/Duplet rule | Octet rule | xviii-electron rule | 32-electron rule |
Every bit a general dominion, a chief-grouping element (except hydrogen or helium) tends to react to form a stwop6 electron configuration. This trend is called the octet rule, because each bonded atom has eight valence electrons including shared electrons. Similarly, a transition metal tends to react to course a dxsouthwardiip6 electron configuration. This tendency is called the 18-electron dominion, considering each bonded atom has xviii valence electrons including shared electrons.
Chemic reactions [edit]
The number of valence electrons in an atom governs its bonding beliefs. Therefore, elements whose atoms can have the same number of valence electrons are grouped together in the periodic table of the elements.
The well-nigh reactive kind of metal element is an alkali metal of group one (e.g., sodium or potassium); this is because such an atom has merely a single valence electron. During the formation of an ionic bail, which provides the necessary ionization energy, this 1 valence electron is easily lost to form a positive ion (cation) with a airtight shell (eastward.g., Na+ or K+). An element of group ii of group 2 (east.g., magnesium) is somewhat less reactive, because each atom must lose two valence electrons to form a positive ion with a airtight shell (e.m., Mg2+).
Within each grouping (each periodic table column) of metals, reactivity increases with each lower row of the table (from a calorie-free element to a heavier chemical element), because a heavier element has more than electron shells than a lighter chemical element; a heavier element'south valence electrons be at higher principal quantum numbers (they are farther abroad from the nucleus of the atom, and are thus at higher potential energies, which ways they are less tightly leap).
A nonmetal cantlet tends to attract additional valence electrons to attain a full valence shell; this can be achieved in one of two ways: An atom can either share electrons with a neighboring atom (a covalent bail), or it can remove electrons from another atom (an ionic bond). The near reactive kind of nonmetal element is a halogen (e.yard., fluorine (F) or chlorine (Cl)). Such an atom has the following electron configuration: s2pfive; this requires only 1 additional valence electron to form a closed trounce. To form an ionic bond, a halogen atom tin can remove an electron from some other atom in guild to course an anion (e.1000., F−, Cl−, etc.). To form a covalent bond, one electron from the halogen and one electron from another atom form a shared pair (e.k., in the molecule H–F, the line represents a shared pair of valence electrons, one from H and i from F).
Within each group of nonmetals, reactivity decreases with each lower row of the table (from a low-cal element to a heavy chemical element) in the periodic table, because the valence electrons are at progressively higher energies and thus progressively less tightly jump. In fact, oxygen (the lightest element in group sixteen) is the well-nigh reactive nonmetal after fluorine, even though it is not a halogen, because the valence shell of a element of group vii is at a higher principal quantum number.
In these simple cases where the octet rule is obeyed, the valence of an cantlet equals the number of electrons gained, lost, or shared in order to course the stable octet. However, in that location are as well many molecules that are exceptions, and for which the valence is less clearly defined.
Electric electrical conductivity [edit]
Valence electrons are also responsible for the electrical conductivity of an element; as a result, an element may be classified as a metal, a nonmetal, or a semiconductor[ clarification needed ] (or metalloid).[ citation needed ]
| 1 | ii | iii | 4 | 5 | 6 | 7 | 8 | 9 | x | xi | 12 | xiii | 14 | xv | 16 | 17 | 18 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group → | ||||||||||||||||||||||||||||||||
| ↓ Period | ||||||||||||||||||||||||||||||||
| 1 | H | He | ||||||||||||||||||||||||||||||
| 2 | Li | Be | B | C | N | O | F | Ne | ||||||||||||||||||||||||
| 3 | Na | Mg | Al | Si | P | S | Cl | Ar | ||||||||||||||||||||||||
| four | K | Ca | Sc | Ti | Five | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr | ||||||||||||||
| 5 | Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe | ||||||||||||||
| 6 | Cs | Ba | La | Ce | Pr | Nd | Pm | Sm | European union | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | Hf | Ta | W | Re | Bone | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn |
| vii | Fr | Ra | Ac | Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og |
| | ||||||||||||||||||||||||||||||||
| Metal Metalloid Nonmetal Unknown properties Background color shows metallic–metalloid–nonmetal tendency in the periodic table | ||||||||||||||||||||||||||||||||
Metallic elements generally have high electrical conductivity when in the solid state. In each row of the periodic tabular array, the metals occur to the left of the nonmetals, and thus a metal has fewer possible valence electrons than a nonmetal. Still, a valence electron of a metal atom has a small ionization energy, and in the solid-land this valence electron is relatively gratis to leave one atom in guild to acquaintance with another nearby. Such a "gratis" electron can exist moved under the influence of an electric field, and its move constitutes an electric current; it is responsible for the electric electrical conductivity of the metal. Copper, aluminium, silver, and gold are examples of good conductors.
A nonmetallic element has low electrical electrical conductivity; it acts as an insulator. Such an element is found toward the correct of the periodic table, and it has a valence shell that is at least half full (the exception is boron). Its ionization energy is large; an electron cannot exit an atom hands when an electric field is practical, and thus such an element can conduct only very small electrical currents. Examples of solid elemental insulators are diamond (an allotrope of carbon) and sulfur.
A solid compound containing metals can likewise be an insulator if the valence electrons of the metallic atoms are used to form ionic bonds. For example, although elemental sodium is a metal, solid sodium chloride is an insulator, considering the valence electron of sodium is transferred to chlorine to form an ionic bail, and thus that electron cannot be moved easily.
A semiconductor has an electrical electrical conductivity that is intermediate betwixt that of a metal and that of a nonmetal; a semiconductor also differs from a metal in that a semiconductor's conductivity increases with temperature. The typical elemental semiconductors are silicon and germanium, each cantlet of which has 4 valence electrons. The properties of semiconductors are all-time explained using band theory, as a consequence of a pocket-sized energy gap betwixt a valence band (which contains the valence electrons at absolute zero) and a conduction band (to which valence electrons are excited by thermal energy).
References [edit]
- ^ Petrucci, Ralph H.; Harwood, William South.; Herring, F. Geoffrey (2002). General chemical science: principles and modern applications (8th ed.). Upper Saddle River, Northward.J: Prentice Hall. p. 339. ISBN978-0-13-014329-7. LCCN 2001032331. OCLC 46872308.
- ^ THE Society OF FILLING 3d AND 4s ORBITALS. chemguide.co.united kingdom
- ^ Miessler Thousand.Fifty. and Tarr, D.A., Inorganic Chemistry (2nd edn. Prentice-Hall 1999). p.48.
- ^ Tossell, J. A. (1 November 1977). "Theoretical studies of valence orbital binding energies in solid zinc sulfide, zinc oxide, and zinc fluoride". Inorganic Chemistry. 16 (11): 2944–2949. doi:10.1021/ic50177a056.
- ^ Chi, Chaoxian; Pan, Sudip; Jin, Jiaye; Meng, Luyan; Luo, Mingbiao; Zhao, Lili; Zhou, Mingfei; Frenking, Gernot (2019). "Octacarbonyl Ion Complexes of Actinides [An(CO)8]+/− (An=Th, U) and the Function of f Orbitals in Metal–Ligand Bonding". Chem. Eur. J. 25 (50): 11772–11784. doi:10.1002/chem.201902625. PMC6772027. PMID 31276242.
External links [edit]
- Francis, Eden. Valence Electrons.
Source: https://en.wikipedia.org/wiki/Valence_electron
Post a Comment for "What are Valence electrons? How do they relate to the periodic table?"